Step 4: Work out your introduction's risk to human health
In Step 4, you need to work out the human health risk of your introduction - is it medium to high, low or very low? To work this out start at 4.1 and continue as far as you need to through each step.
Once you have your answer for human health, move to step 5 to work out the risk to the environment of your introduction.
At Step 6, you'll combine the human health risk and environment risk for the final category of your introduction.
To be able to finish your categorisation you need to work out the risks of your introduction to human health and the environment.
Step 4.1 Introductions that are always medium to high risk for human health
Instructions
Go through A, B and C to work out if you are, or are not, introducing any of these types of chemicals. You must keep records of study reports and other information that you used to answer each question.
B. Is your chemical a certain polyhalogenated organic chemical?.
C. Is your chemical a certain chemical at the nanoscale?.
_______________________________________________________________________________________________________________________________
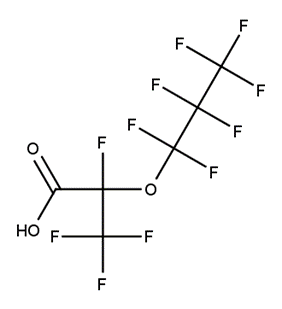
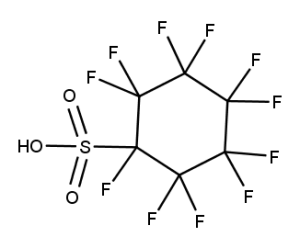
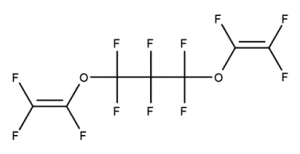
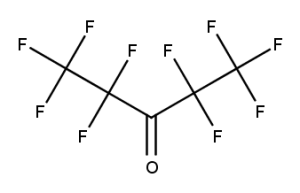
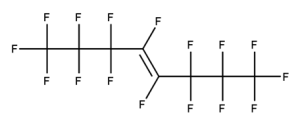
A. Is your chemical a designated fluorinated chemical (including per- and polyfluoroalkyl substances, known as PFAS)?
Fluorinated chemicals contain fluorine atoms and include per- and polyfluoroalkyl substances (PFAS). These are commonly used in products to add resistance to heat, other chemicals, and abrasion. They also act as dispersion, wetting or surface treatment agents. We have an increased level of concern for introductions of designated fluorinated chemicals (including PFAS), because these chemicals, or their degradation products, may be persistent in the environment, bioaccumulate and be highly toxic.
A designated fluorinated chemical is an industrial chemical that contains a sequence of atoms (whether linear, branched or cyclic) to which all of the following applies:
- subject to paragraph (b), the sequence consists only of at least 4, but no more than 20, fluorinated carbon atoms, none of which are fluorinated carbon atoms that are part of conjugated double bonds;
- if the sequence is broken in any place, the break consists only of a single atom or single substituted atom;
- the sequence includes at least one perfluorinated methyl group (CF3) or perfluorinated methylene group (CF2).
Fluorinated carbon atom means a carbon atom attached to at least one fluorine atom.
For a chemical to meet the definition of a ‘designated fluorinated chemical’:
• the sequence of carbon atoms can be linear, branched, or cyclic.
• the sequence must consist only of at least 4, but maximum 20 fluorinated carbon atoms.
• the sequence can include multiple breaks by a single atom (such as O or S) or substituted atom (such as C=O)
• the chemical can be polyfluorinated, provided the sequence contains at least one perfluorinated methyl group (CF3) or perfluorinated methylene group (CF2)
• fluorinated carbon atoms that are part of conjugated double bonds are not counted as part of the sequence.
Conjugated double bonds could include aromatic carbons.
We have extra guidance on categorisation of fluorinated chemicals
Some introductions are always medium to high risk to human health. This means they will be in the assessed introduction category and you need to apply for an assessment certificate.
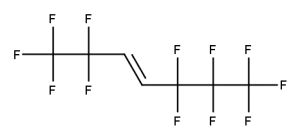
Example 1
CAS number: 13252-13-6
Notes about this chemical:
- Sequence of 5 fluorinated carbon atoms.
- Ether linkage is a single atom break in the sequence.
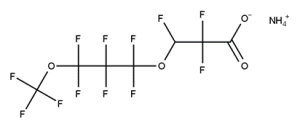
Example 2
CAS number: 2106-55-0
Notes about this chemical:
- Sequence of 6 fluorinated carbon atoms.
- Sequence can be cyclic.
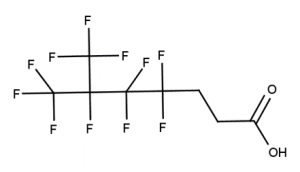
Example 3
CAS number: 13846-22-5
Notes about this chemical:
- Sequence of 7 fluorinated carbon atoms.
- Fluorinated carbon atoms on double bond considered part of sequence as double bond is not conjugated.
- Ether linkages considered a single atom break in the sequence.
- Multiple single atom or single substituted atom breaks allowed in the sequence.
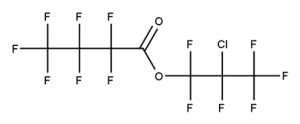
Example 4
CAS number: 684-32-2
Notes about this chemical:
- Sequence of 4 fluorinated carbon atoms.
- Carbon atom of carbonyl group considered a single substituted atom break in the sequence.
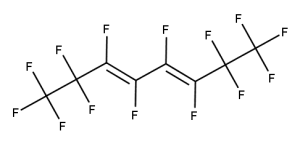
Example 5
CAS number: 115264-42-1
Notes about this chemical:
- Sequence of 8 fluorinated carbon atoms.
- Fluorinated carbon atoms on double bond considered part of sequence as the double bond is not conjugated.
Example 6
CAS number: 958445-44-8
Notes about this chemical:
- Sequence of 6 fluorinated carbon atoms.
- “CFH” carbon atom included in sequence.
- Ether linkages considered a single atom break in the sequence.
- Multiple single atom or single substituted atom breaks allowed in the sequence.
Example 7
CAS number: 207987-90-4
Notes about this chemical:
- Sequence of 5 fluorinated carbon atoms.
- Sequence can be branched.
Example 1
CAS number: 54326-26-0
Notes about this chemical:
- Sequence of 3 fluorinated carbon atoms
- Fluorinated carbon atoms on aromatic ring excluded from sequence
Example 2
CAS number: 105311-63-5
Notes about this chemical:
- Sequence of 2 fluorinated carbon atoms
- Sequence is broken by fluorinated carbon atoms that are part of conjugated double bonds
Example 3
CAS number: 2511100-75-5
Notes about this chemical:
- Sequence of 3 fluorinated carbon atoms
- Sequence is broken by more than a single atom or single substituted atom
Example 4
CAS number: 67135-90-4
Notes about this chemical:
- Sequence of 3 fluorinated carbon atoms
- Sequence broken by more than a single atom or single substituted atom
No I am not introducing a designated fluorinated chemical
You must have information about your chemical's identity as proof that you're not introducing this type of chemical. You (or the chemical identity holder) need to provide the information if we ask for it.
Next step: Go to 'B. Is your chemical a certain polyhalogenated organic chemical?'.
Yes I am introducing a designated fluorinated chemical
Outcome: Your introduction is medium to high indicative risk to both human health and the environment. This means your introduction is in the assessed category and called an 'assessed introduction'.
- Before you can introduce the chemical, you must apply for an assessment certificate and select 'Health and environment focus' as the application type or apply for a commercial evaluation authorisation (if you meet the strict criteria).
- When you apply for an assessment certificate, you need to answer ‘yes’ when we ask if your introduction is a specified class of introduction. When we receive your application, we’ll contact you to ask for extra information that we need to assess the risks of your introduction.
__________________________________________________________________________________________________________________________________
B. Is your chemical a certain polyhalogenated organic chemical?
Polyhalogenated organic chemicals are carbon-based chemicals that contain more than one covalently bonded halogen atom, such as bromine, chlorine, fluorine, or iodine. Polyhalogenated organic chemicals are commonly used as flame retardants in plastics, textiles, and electronic circuitry. They may have long-term effects on human health and the environment. We have an increased level of concern for introductions of chemicals that are polyhalogenated organic chemicals because these chemicals, or their degradation products, may be persistent in the environment, bioaccumulate and be highly toxic.
No I am not introducing this type of chemical
You must have information about your chemical's identity as proof that you're not introducing this type of chemical. You (or the chemical identity holder) need to provide the information if we ask for it.
Next step: Go to 'C. Is your chemical a certain chemical at the nanoscale?' below.
Yes I am introducing this type of chemical
All introductions of polyhalogenated chemicals are specified class of introduction.
If the chemical identity information that you (or the chemical identity holder) have confirms you are introducing this type of chemical, you must consider which of the following circumstances apply to your introduction.
1. Polyhalogenated organic chemicals introduced at volumes less than or equal to 100 kg each year
Next step: Go to 'C. Is your chemical a certain chemical at the nanoscale?' below.
2. Polyhalogenated organic chemicals introduced at volumes higher than 100 kg each year
You need to have test results about the persistence of your chemical and any of its known environmental degradation products.
- Known environmental degradation products refer to the expected breakdown products of the chemical under environmentally relevant conditions. These breakdown products are ones that have been found in studies or reported in scientific literature.
- A persistent chemical remains intact in the environment for long periods of time. A chemical is persistent if its degradation half-life (T1/2) is greater than or equal to:
- 2 days in air or
- 2 months in water or
- 6 months in soil or
- 6 months in sediment.
To prove that your chemical and any of its known environmental degradation products are not persistent, we accept study results in option 1 or 2.
Option 1
A study conducted following an acceptable test guideline for ready biodegradability that results in the pass levels being reached within one of the following time periods:
- specified time period – such that the chemical is considered to be readily biodegradable or
- duration of the test – but not within the specified time period for the chemical to be considered readily biodegradable, provided biodegradation has started within the specified time period
If you have this study showing these results, then move on to 'C. Is your chemical a certain chemical at the nanoscale?' below.
Option 2
A study conducted following an acceptable test guideline for Transformation in Aquatic Sediment Systems that results in both a degradation half-life of less than 2 months in water and 6 months in sediment.
If you have this study showing these results, then move on to 'C. Is your chemical a certain chemical at the nanoscale?' below.
If you do not have either of the study results described in option 1 or 2
Outcome: Your introduction is medium to high indicative risk to human health and the environment because you cannot prove that your chemical (and any of its known environmental degradation products) are not persistent. This means your introduction is in the assessed category and called an 'assessed introduction'.
- Before you can introduce the chemical, you must apply for an assessment certificate and select 'Health and environment focus' as the application type, or apply for a commercial evaluation authorisation (if you meet the strict criteria).
- When you apply for an assessment certificate, you need to answer ‘yes’ when we ask if your introduction is a specified class of introduction. When we receive your application, we’ll contact you to ask for extra information that we need to assess the risks of your introduction.
______________________________________________________________________________________________________________________________
C. Is your chemical a certain chemical at the nanoscale?
Introductions of chemicals that meet all 4 criteria below are medium to high indicative risk to both human health and the environment. We refer to these introductions as 'certain chemicals at the nanoscale'. We have an increased level of concern for chemicals at the nanoscale, because of uncertainty about the risks of some of these chemicals due to their potentially different properties, such as chemical reactivity, relative to the non-nanoscale forms of the chemicals.
- It is introduced as a solid or is in a dispersion.
- It consists of solid particles in an unbound state or as an aggregate or agglomerate. At least 50% (by number size distribution) of the particles have at least 1 external dimension in the particle size range of 1nm to 100nm (ie. the nanoscale). Note that if you meet criteria 1 and 2, and regardless of whether you meet criteria 3 and 4, your introduction is a specified class of introduction.
- It is not soluble. This means the solubility of the chemical in water is less than 33.3 g/L measured following OECD test guideline 105 or 120 for water solubility; or the dissolution rate of the chemical is not more than 70%.
- The introduction of the nanoscale portion of the chemical (the part that has a particle size range of 1nm to 100nm) is not incidental to the introduction of the non-nanoscale portion. This is the case if any of the following apply:
- the manufacture of the chemical (in Australia or overseas) at the nanoscale is the result of a deliberate manufacturing decision
- the manufacture of the chemical (in Australia or overseas) at the nanoscale is necessary for the manufacture of the non-nanoscale portion of the chemical. This means that to make the non-nanoscale chemical, part of the chemical has to be at the nanoscale
- the chemical at the nanoscale has specific technical characteristics that are the intended result of changes in the manufacturing process. For example, if the process of manufacturing the chemical changes in order to change the particle size of the chemical, or its properties at the nanoscale. This could happen by:
- mechanical actions like milling, grinding, shearing, sieving or sonication
- chemicals reactions like electrochemical exfoliation, or catalysts
- other changes such as changes to pressure or temperature or pH or solvent
Yes I am introducing this type of chemical
This means that your introduction meets all 4 criteria above and is a 'certain chemical at the nanoscale'.
Outcome: Your introduction has a medium to high indicative risk to both human health and the environment. This means your introduction is in the assessed category and called an ‘assessed introduction’.
- Before you can introduce the chemical, you must apply for an assessment certificate and select 'Health and environment focus' as the application type or apply for a commercial evaluation authorisation (if you meet the strict criteria).
- When you apply for an assessment certificate, you need to answer ‘yes’ when we ask if your introduction is a specified class of introduction. When we receive your application, we’ll contact you to ask for extra information that we need to assess the risks of your introduction.
No I am not introducing this type of chemical
This means that you have information or studies to prove that your chemical does not meet any of the 4 criteria, or it only meets some of the 4 criteria. Answering the questions below will help you prove this. As you go through the questions, we'll tell you the next steps you should take.
Definition - specified class of introduction
A ‘specified class of introduction’ are introductions that have an increased level of concern to human health or the environment. The reason is due to greater potential for certain hazards or high level of human or environmental exposure. Additional, or different, requirements relating to hazard information, reporting or record keeping apply to specified classes of introductions. These vary depending on whether you have categorised your introduction as exempted, reported or assessed.
Next – Step 4.2 Introductions that can be low risk for human health
Step 4.2 Introductions that can be low risk for human health
This step relates to introductions that are internationally assessed for human health. These must meet all of the following criteria to be considered ‘low indicative risk’ for human health.
Skip this step if you are not using an internationally assessed chemical.
Note: Your introduction might still be low indicative risk for human health but you will need to complete steps 4.3, 4.4 and 4.5 to work this out.
Step 4.2.1
Refer to our Guide to categorising internationally assessed introductions. It has extra information for introducers using international assessments and covers scenarios and outcomes for chemicals that are internationally assessed for:
- human health only
- the environment only
- both human health and the environment
It also lists the trusted overseas bodies we accept assessments from.
The relevant section to refer to in the guide to help you complete step 4.2 is Internationally assessed for human health only.
Step 4.2.2
Once you’ve read the guide to categorising internationally assessed introductions, you'll be able to work out whether your introduction either:
- meets our criteria for internationally assessed for human health
- does not meet our criteria for internationally assessed for human health
Your introduction meets our criteria for internationally assessed for human health
Option 1
- Keep the outcome you already have — your introduction is low risk for human health; and
- Go to step 5 to start categorising your introduction's indicative risk for the environment.
Option 2
Check to see if your introduction can be very low risk for human health by completing the rest of step 4:
- Complete Step 4.3 — Work out your introduction's human health exposure band; then
- Complete Step 4.4 — Work out your introduction's human health hazard characteristics
Once you’ve done this, go to step 4.5 for your final answer.
Your introduction does not meet our criteria for internationally assessed for human health
Continue with step 4 to work out your introduction's risk for human health.
Next:
- Complete Step 4.3 — Work out your introduction's human health exposure band; then
- Complete Step 4.4 — Work out your introduction's human health hazard characteristics
Once you’ve done this, go to step 4.5 for your final answer.
If you've established your introduction is NOT medium to high risk for human health (Step 4.1), now see if your introduction CAN be low risk for human health.
Step 4.3 Work out your human health exposure band
Why do you need to work out your introduction's exposure band?
It is part of the process to identify the indicative human health risk of your introduction. In step 5, you also have to work out your introduction's environment exposure band.
What does a human health exposure band identify about your introduction?
It identifies the likelihood and extent of human exposure to the chemical. This likelihood and extent of exposure increases with each band. Exposure band 4 is the highest exposure band. Introductions in human health exposure band 4 will have the highest level of human exposure.
Information that's used to assign a chemical to its correct exposure band
The information you need to be able to work out your exposure band can be different depending on the exposure band criteria you will be using. Some of the exposure band criteria mainly depend on human health categorisation volume, while others mainly depend on the concentration of your chemical when it's introduced into Australia and during its end use. This is a full list of the information you might need to be able to work out your human health exposure band:
- Human health categorisation volume (needed for scenario 1 of Exposure Band 2, scenario 1 of Exposure Band 3 and Exposure Band 4) - use this guidance to help you calculate human health categorisation volume
- Concentration of your chemical at introduction (needed for Exposure Band 1, scenario 2 of Exposure Band 2 and scenario 2 of Exposure Band 3).
- Concentration of your chemical across all end uses (needed for Exposure Band 1, scenario 2 of Exposure Band 2 and scenario 2 of Exposure Band 3).
- If it has an end use in tattoo inks (which is always in human health exposure band 4)
- If there are any consumer end uses for the chemical introduction (needed for Exposure Band 1 and scenario 2 of Exposure Band 2 and).
Are you introducing a chemical with an end use in tattoo inks?
If yes, your introduction is in human health exposure band 4 – skip to Step 4.4: Work out your human health hazard characteristics.
If no, continue below to work out which exposure band (1, 2, 3 or 4) applies to your introduction.
Work out your human health exposure band
Exposure band 1 criteria
Your introduction is in exposure band 1 if it is either Scenario 1 or Scenario 2.
Scenario 1 – not for consumer end use and less than 0.1% concentration
Your introduction must meet all criteria below:
- The concentration of your chemical at introduction is less than 0.1 %.
- The concentration of your chemical is less than 0.1 % across all your introduction’s end uses.
- Your introduction is not for any consumer end use.
If you meet all criteria for Scenario 1, your introduction is in exposure band 1 for human health. Next, choose between option 1 and option 2:
Option 1: The indicative risk of your introduction to human health is low risk. You can skip the remainder of step 4 and go to step 5 to work out your introduction’s risk to the environment. Once you have completed step 5, you will be able to work out your introduction category at step 6.
Option 2: You can choose to continue with the rest of step 4 to see if your introduction can be very low risk to human health. To do this, work through Step 4.4: Work out your human health hazard characteristics, followed by step 4.5. You must then complete step 5 to work out your introduction’s risk to the environment.
---------------------------------------------------
If your introduction is not scenario 1, go to scenario 2 or exposure band 2 criteria.
Scenario 2 – controlled introduction and use of a chemical
Your introduction must meet all criteria below:
- Your introduction of the industrial chemical is not of an industrial chemical that has an end use in tattoo inks.
- The human health categorisation volume of your chemical does not exceed 25 kg.
- Your introduction is not for any consumer end use.
- During introduction and use of your chemical, you will implement one or both control measures:
1. isolate the industrial chemical from any person who could be exposed to it
2. use engineering controls to eliminate or minimise exposure to people, including a mechanical device or process.- If people could still be exposed to the chemical even after implementing the control measures above, then you will implement other control measures to minimise potential exposure as far as reasonably practicable. This must include workers wearing suitable personal protective equipment that you provide.
- You (the introducer) have full control of the industrial chemical.
See example below of a controlled introduction and use of a chemical that meets criteria for human health exposure band 1.
If you meet criteria for Scenario 2, your introduction is in exposure band 1 for human health. Next, choose between option 1 and option 2:
Option 1: The indicative risk of your introduction to human health is low risk. You can skip the remainder of step 4 and go to step 5 to work out your introduction’s risk to the environment. Once you have completed step 5, you will be able to work out your introduction category at step 6.
Option 2: You can choose to continue with the rest of step 4 to see if your introduction can be very low risk to human health. To do this, work through Step 4.4: Work out your human health hazard characteristics, followed by step 4.5. You must then complete step 5 to work out your introduction’s risk to the environment.
---------------------------------------------------
If your introduction is not scenario 2, go to the exposure band 2 criteria.
Exposure Band 2 criteria
Your introduction is in exposure band 2 if it is either Scenario 1 or Scenario 2:
Scenario 1
The human health categorisation volume of your chemical does not exceed 25kg.
Scenario 2
Your introduction must meet all of the following criteria:
- The concentration of your chemical at introduction is less than 0.1 %.
- The concentration is less than 0.1 % across all your introduction’s end uses.
- The introduction has at least 1 consumer end use.
If you meet criteria for either Scenario 1 or 2, your introduction is in exposure band 2 for human health – skip to Step 4.4: Work out your human health hazard characteristics.
Otherwise, go to Exposure band 3 criteria.
Exposure band 3 criteria
Your introduction is in exposure band 3 if it is either Scenario 1 or Scenario 2:
Scenario 1
- The human health categorisation volume for your chemical does not exceed 100 kg - use this guidance to calculate.
Scenario 2
You must meet all following criteria:
- The concentration of your chemical at introduction is less than or equal to 1%.
- The concentration is less than or equal to 1% across all your introduction’s end uses .
If you meet criteria for Scenario 1 or 2, your introduction is in human health exposure band 3 – skip to Step 4.4: Work out your human health hazard characteristics.
Otherwise, go Exposure band 4 criteria.
Exposure band 4 criteria
Your introduction is in exposure band 4 if it is either Scenario 1 or Scenario 2:
Scenario 1
Your chemical will have an end use in tattoo inks.
Scenario 2
The human health categorisation volume for your introduction is greater than 100 kg.
If you meet criteria for Scenario 1 or 2, your introduction is in human health exposure band 4 – next, go to Step 4.4: Work out your human health hazard characteristics.
What is a 'consumer end use'?
Step 4.4 Work out your human health hazard characteristics
Important! Your starting point is always hazard band C (the highest hazard band)
Then work your way down the hazard bands (that is, C then B then A) to check if your chemical has any characteristics in these bands. After reading this page, go to human health hazard band C hazard characteristics.
You must have permission to use information that you relied on to demonstrate the absence of hazard characteristics. If we ask you for the information that you relied on to categorise your introduction, you need to provide us with the detailed information, including full study reports, of the kind we specify in this step to demonstrate the absence of the hazard characteristics.
Hazard characteristics in human health hazard bands
A chemical has a human health hazard characteristic if the chemical can cause damage, harm or adverse effects to humans. For example, a chemical that has the 'skin corrosion' hazard characteristic can cause irreversible damage to the skin of humans.
Human health hazard characteristics are split up into hazard bands. Hazard characteristics of most concern are in hazard band C, while those of lower concern are in hazard band A.
Our pages for human health hazard bands C, B and A describe hazard characteristics (eg carcinogenicity and so on) in each hazard band and the information you need to have to prove your chemical does not have a particular hazard characteristic.
Information you need and hazard characteristics you need to consider
This varies depending on your introduction’s human health exposure band.
If your introduction is in a lower exposure band
Generally, in the lower exposure bands, where the level of exposure to humans is relatively low, as a minimum you have to consider only a few hazard characteristics and you don’t need much information on them.
If your introduction is in a higher exposure band
In comparison, in higher exposure bands, where the level of exposure to humans is higher, generally you’ll need to consider more hazard characteristics and need more information on them.
Information you need for lower indicative risk
You will need more hazard information to be able to get to lower indicative risk outcomes. Generally, within any given human health exposure band you need:
- less hazard information to get to medium to high risk
- more hazard information to get to low risk
- the most hazard information to get to very low risk
See Step 4.5 for more information about indicative human health risk outcomes
Work out if your chemical has any hazard characteristics in human health hazard band A, B, C.
Where to start and when you can stop considering your chemical's hazard characteristics
You must consider each hazard characteristic in the hazard band you are in (unless there is a reason for you to stop sooner) — does your chemical have that hazard characteristic or not?
When you might not need to consider all of the hazard bands
- Because your introduction’s human health exposure band (which you worked out in step 4.3) doesn’t require it. For example, if your introduction’s human health exposure band is 1, you only need to consider the hazards in hazard band C to get to an indicative human health risk of either low or very low.
- Because the outcome for indicative human health risk that you are trying to get to doesn’t require it. For example, if your introduction’s human health exposure band is 3 and you want to get to an indicative human health risk of low, you only need to consider the hazard characteristics in human health hazard band C.
In many cases, you’ll only need to consider hazard band C. But in other cases you might need to consider C, B and A, because your introduction is in exposure band 3 or 4 and you are trying to get to very low indicative human health risk.
See step 4.5 for more about indicative human health risk outcomes
When you can stop working through your chemical’s human health hazard characteristics
Stop if you:
- determine that your chemical has a hazard characteristic in the hazard band (eg carcinogenicity — you are in hazard band C) or
- cannot demonstrate that your chemical does not have a certain hazard characteristic in that hazard band. This means that we consider your chemical to have this hazard characteristic or
- get to an indicative human health risk outcome and don’t want to go any further — see step 4.5 for more information about human health risk outcomes or
- have demonstrated that your chemical does not have any hazard characteristics in hazard bands C, B and A. This would only be needed for human health exposure bands 3 and 4. It means that the indicative human health risk of your introduction is very low.
After you stop, you don’t need to consider the remaining hazard characteristics in the hazard band where you stopped, or any of the hazard characteristics in lower hazard bands. Take note of where and why you stopped, then move to step 4.5.
Example: Anna's introduction is in human health exposure band 4. She considers all of the hazard characteristics in human health hazard band C and can demonstrate that her chemical does not have any of these hazards. Anna then moves on to hazard band B. She works through the hazard characteristics in this hazard band in the order that they are shown in the table. When Anna comes to eye damage, she finds that her chemical has the 'eye damage' hazard characteristic. This means Anna can stop there. The indicative human health risk of Anna's introduction is medium to high. She does not need to continue further to see if her chemical has any of the other hazard characteristics in hazard band B, like skin sensitisation, or specific target organ toxicity after repeated exposure. Also Anna doesn't need to consider if her chemical has any of the hazard characteristics in hazard band A, such as skin irritation.
How to consider each hazard characteristic
Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there and move to step 4.4.
If it does not meet the hazard characteristic definition, you’ll need to try and prove that your chemical does not have this hazard characteristic.
Our pages on hazard bands C, B and A describe hazard characteristics and the ways to prove that your chemical does not have a hazard characteristic.
How to prove that your chemical does not have a hazard characteristic
You can read about your options to prove that your chemical does not have a particular hazard characteristic on each human health hazard band page. These options include
- checking if your chemical is on the list of chemicals with high hazards for categorisation
- in silico predictions
- in vitro test results
- in vivo test results
- suitable read-across information in place of information on the chemical itself
- other information about your chemical that means that testing and in silico predictions are not necessary (that is, information waivers)
If you have access to existing information on the chemical or suitable read-across information, you should consider these first. If you need to generate new data to prove the absence of a hazard characteristic, you should choose non-animal test data when possible. You should only generate new animal test data as a last resort.
See our section on use of animal test data
If you can prove that your chemical does not have the hazard characteristic, move on to the next hazard characteristic in that hazard band, or from the next hazard band down.
If you cannot prove that your chemical does not have the hazard characteristic, stop there – your chemical is considered to have this hazard characteristic. Take note of the hazard band that this hazard characteristic is in and move on to step 4.5.
If your chemical is one of these:
- polyhalogenated organic chemical
- UV filter
- is introduced for an end use in a tattoo ink
- is introduced for an end use in an article that is a children’s toy or a children’s care product
- is introduced for an end use in an article with food contact
there may be different requirements for you to prove that your chemical does not have certain hazard characteristics.
In most cases we do not expect that you will have information about the high level hazard characteristics in human health hazard band C, such as carcinogenicity. Instead, to demonstrate that your chemical does not have these hazard characteristics you can search the list of chemicals with high hazards for categorisation. This is a list that we have compiled directly from trusted international sources, and provides you with a single place to search for your chemical to check if it might be known to have these hazards. Our hazard band pages tell you when you might need to search the high hazards list.
A list of resources to help you with this step
- List of chemicals with high hazards for categorisation
- In silico information- an overview of which human health and environment (step 5.4) characteristics have in silico options and which in silico models are appropriate.
- Acceptable test guidelines for each human health hazard characteristic listed in this step
- Suitable read across information
- Decision tools for step 4.4 (self-guided tools to help you categorise your introduction):
Human health hazard bands - what are the hazard characteristics in each hazard band?
The following pages describe the hazard characteristics in each hazard band and the information you need to have to prove your chemical does not have a particular hazard characteristic. Follow instructions on each of these pages. Always start with hazard band C.
Human health hazard band C hazard characteristics
Human health hazard characteristics are split into hazard bands. Hazard characteristics of most concern are in hazard band C, while those of lower concern are in hazard band A.
Getting started
Hazard band C has 7 hazard characteristics you need to consider:
- chemical is an inorganic arsenic compound
- chemical contains beryllium, cadmium, chromium (VI), lead, or nickel
- carcinogenicity
- reproductive toxicity
- developmental toxicity
- adverse effects mediated by an endocrine mode of action
- genetic toxicity
Work your way through each hazard characteristic on this page. Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there - your introduction's human health hazard band is C. Move on to the next step - step 4.5 Work out your human health risk for categorisation.
If it does not meet the hazard characteristic definition, you’ll need to prove that your chemical does not have this hazard characteristic. The information that you need to prove this for each hazard characteristic is shown below. If you do not have this information, stop there - your introduction’s human health hazard band is C. Move onto the next step – step 4.5 Work out your human health risk for categorisation.
If you do have this information (so you can prove that the chemical does not have the hazard characteristic), move onto the next hazard characteristic on this page.
After you have considered all the hazard characteristics on this page and have proven that the chemical does not have any of them, decide whether you can stop there or continue to human health hazard band B. This depends on the exposure band of your introduction.
If your introduction is in human health exposure band 1 or 2, stop here - you don’t need to consider any other hazard characteristics. Next go to step 4.5 to work out your human health risk for categorisation.
If your introduction is in human health exposure band 3 you can choose to stop (and go to step 4.5 to work out your human health risk for categorisation), or to continue to human health hazard band B and then A.
If your introduction is in human health exposure band 4, continue to human health hazard band B.
Detailed instructions about each human health hazard band C hazard characteristics you need to consider
Chemical is an inorganic arsenic compound
A chemical is an inorganic arsenic compound means both of these apply to the industrial chemical:
- contains arsenic and
- does not contain carbon.
There are no extra information requirements to prove that the chemical does not have this hazard characteristic.
Chemical contains beryllium, cadmium, chromium (VI), lead or nickel
Chemical contains beryllium, cadmium, chromium (VI), lead or nickel means that the industrial chemical contains one or more of the following chemical elements:
- beryllium
- cadmium
- chromium (VI)
- lead
- nickel
There are no extra information requirements to prove that the chemical does not have this hazard characteristic.
Carcinogenicity
Carcinogenicity means that any of the following apply to the industrial chemical:
- the chemical is a known, presumed or suspected human carcinogen, as described in chapter 3.6 of the GHS, with the chemical classified as carcinogenicity (category 1 or 2), or
- the chemical is on the list of chemicals with high hazards for categorisation based on its carcinogenicity, or
- the chemical is an ester or salt of specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation based on carcinogenicity, unless an exception as identified in the table below is met for that chemical, or
- an in vivo study on the chemical conducted following an acceptable test guideline for carcinogenicity, chronic toxicity, subchronic oral toxicity, subchronic dermal toxicity or subchronic inhalation toxicity results in the induction of cancer, or an increase in the incidence of cancer.
Information required to demonstrate the absence of the hazard characteristic, carcinogenicity
- Confirmation that the chemical is not on the list of chemicals with high hazards for categorisation, based on its carcinogenicity and
- Confirmation that the chemical is not an ester or salt of the specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation, based on carcinogenicity
Carcinogenicity - exception criteria
Check the following table – an ester or salt of the chemical has the carcinogenicity characteristic, unless one or more of the following exception criteria apply.
| CAS number | Chemical name | An ester or salt of the chemical has the carcinogenicity hazard characteristic, unless one or more of the below exception criteria apply |
|---|---|---|
| 108-78-1 | 1,3,5-Triazine-2,4,6-triamine (Melamine) |
|
| 139-13-9 | Glycine, N,N-bis(carboxymethyl)- |
|
| 90-43-7 | [1,1’-Biphenyl]-2-ol |
|
| 615-05-4 | 1,3-Benzenediamine, 4-methoxy- (diaminoanisole) |
|
Note – Low levels of low molecular weight species (in relation to a polymer) means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol.
- In addition, if the human health exposure band for the introduction is 4 and the chemical is a UV filter, information is required to justify why the chemical would not cause carcinogenicity mediated by exposure to UV light. This may include one or more of the following:
- the chemical has a molar extinction/absorption coefficient of less than 1,000 Lmol- 1cm-1 at wavelengths between 290 and 700 nm (based on the results of a study following OECD test guideline 101), or
- results from in vitro phototoxicity studies, or
- results from in vivo carcinogenicity studies where the methods have been modified to include photoactivation.
More information: categorisation of UV filters
This page accompanies step 4.4 Work out your human health hazard characteristics.
Reproductive toxicity
Reproductive toxicity means that any of the following apply to the industrial chemical:
- the chemical is known, presumed or suspected to produce adverse effects on sexual function and fertility, as described in chapter 3.7 of the GHS, with the chemical classified as toxic to reproduction (category 1 or 2), or
- the chemical is on the list of chemicals with high hazards for categorisation based on its reproductive toxicity, or
- the chemical is an ester or salt of specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation based on reproductive toxicity, unless an exception as identified in the table below is met for that chemical, or
- an in vivo study on the chemical conducted following an acceptable test guideline for reproductive toxicity, carcinogenicity, chronic toxicity, subchronic oral toxicity, subchronic dermal toxicity or subchronic inhalation toxicity results in adverse effects on sexual function and fertility, as described in chapter 3.7 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, reproductive toxicity
- If the chemical is a polyhalogenated organic chemical and the human health exposure band for the introduction is 4 -
- an in vivo test result on the chemical or suitable read across information conducted following an acceptable test guideline for reproductive toxicity, which results in none of the adverse effects on sexual function or fertility described in chapter 3.7 of the GHS;
- Otherwise –
- confirmation that the chemical is not on the list of chemicals with high hazards for categorisation, based on its reproductive toxicity
- confirmation that the chemical is not an ester or salt of the specified chemicals shown in the below table, which are on the list of chemicals with high hazards for categorisation based on reproductive toxicity.
Reproductive toxicity - exception criteria
Check the following table - an ester or salt of the chemical has the reproductive toxicity hazard characteristic, unless one or more of the following exception criteria apply.
| CAS name | Chemical name | An ester or salt of the chemical has the reproductive toxicity hazard characteristic, unless one or more of the below exception criteria apply |
|---|---|---|
| 110-80-5 | Ethanol, 2-ethoxy-, |
|
| 109-86-4 | Ethanol, 2-methoxy- |
|
| 98-73-7 | Benzoic acid, 4-(1,1-dimethylethyl)- |
|
| 97-99-4 | 2-Furanmethanol, tetrahydro- (tetrahydro-2-furylmethanol) |
|
| Various | Boric acid |
|
| 80-05-7 | Phenol, 4,4'-(1-methylethylidene)bis- (Bisphenol A) |
|
| 80-09-1 | Phenol, 4,4'-sulfonylbis- (Bisphenol S) |
|
| 98-54-4 | Phenol, 4-(1,1-dimethylethyl)- |
|
| Various | Nonylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
| Various | Dodecylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
Note – Low levels of low molecular weight species (in relation to a polymer) means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol.
Developmental toxicity
Developmental toxicity means that any of the following apply to the industrial chemical:
- the chemical is known, presumed or suspected to produce adverse effects on the development of the offspring or effects on the offspring via lactation, as described in chapter 3.7 of the GHS, with the chemical classified as follows:
- toxic to reproduction (category 1 or 2), or
- effects on or via lactation, or
- the chemical is on the list of chemicals with high hazards for categorisation based on its developmental toxicity, or
- the chemical is an ester or salt of specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation based on developmental toxicity, unless an exception as identified in the table below is met for that chemical, or
- an in vivo study on the chemical conducted following an acceptable test guideline for developmental toxicity or reproductive toxicity results in adverse effects on the development of the offspring or effects on the offspring via lactation, as described in chapter 3.7 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, developmental toxicity
- If the chemical is a polyhalogenated organic chemical and the human health exposure band for the introduction is 4 –
- an in vivo test result on the chemical or suitable read across information conducted following an acceptable test guideline for developmental toxicity or reproductive toxicity which results in none of the adverse effects on the development of the offspring or effects on the offspring via lactation, as described in chapter 3.7 of the GHS
- Otherwise –
- confirmation that the chemical is not on the list of chemicals with high hazards for categorisation, based on its developmental toxicity and
- confirmation that the chemical is not an ester or salt of the specified chemicals shown in the below table, which are on the list of chemicals with high hazards for categorisation, based on developmental toxicity.
Developmental toxicity - exception criteria
Check the following table - an ester or salt of the chemical has the development toxicity hazard characteristic, unless one or more of the following exception criteria apply.
| CAS number | Chemical name | An ester or salt of the chemical has the developmental toxicity hazard characteristic, unless one or more of the below exception criteria apply |
|---|---|---|
| 149-57-5 | Hexanoic acid, 2-ethyl (2-EHA) |
|
| 104-76-7 | 1-Hexanol, 2-ethyl- |
|
| 69-72-7 | Benzoic acid, 2-hydroxy- (salicylic acid) |
|
| 110-80-5 | Ethanol, 2-ethoxy-, |
|
| 109-86-4 | Ethanol, 2-methoxy- |
|
| 111-77-3 | Ethanol, 2-(2-methoxyethoxy)- |
|
| 97-99-4 | 2-Furanmethanol, tetrahydro- (tetrahydro-2-furylmethanol) |
|
| Various | Boric acid |
|
| 80-09-1 | Phenol, 4,4'-sulfonylbis- (Bisphenol S) |
|
| Various | Nonylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
Note – Low levels of low molecular weight species (in relation to a polymer) means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol
Adverse effects mediated by an endocrine mode of action
Adverse effects of mediated by an endocrine mode of action means that any of the following apply to the industrial chemical:
- the chemical meets all of the following:
- it shows an adverse effect in an intact organism or its progeny, which is a change in the morphology, physiology, growth, development, reproduction or lifespan of an organism, system or (sub)population that results in an impairment of functional capacity, an impairment of the capacity to compensate for additional stress or an increase in the susceptibility to other influences, and
- it has an endocrine activity, which is the capacity to alter the function(s) of the endocrine system, and
- the adverse effect is a consequence of the endocrine activity
or
- the chemical is on the list of chemicals with high hazards for categorisation, based on its adverse effects mediated by an endocrine mode of action or
- the chemical is an ester or salt of specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation based on adverse effects mediated by an endocrine mode of action unless an exception, as identified in the table below, is met for that chemical, or
- the chemical meets all of the following:
- information is available that is relevant to determining whether the chemical has the hazard characteristic, adverse effects mediated by an endocrine mode of action, and
- the information has been considered in a weight of evidence analysis based on the following guidance documents:
- the EU guidance for identifying endocrine disruptors*, and
- the guidance provided in OECD GD 150**; and
- the weight of evidence analysis concludes that the chemical has the hazard characteristic, adverse effects mediated by an endocrine mode of action.
Information required to demonstrate the absence of the hazard characteristic, adverse effects mediated by an endocrine mode of action
- If the chemical has existing information relevant to determining whether it has the hazard characteristic, adverse effects mediated by an endocrine mode of action, information is required to demonstrate that the chemical does not have this hazard characteristic:
- this must involve a documented weight of evidence analysis based on the EU guidance for identifying endocrine disruptors* and the guidance in OECD GD 150**, and
- the analysis must conclude that the chemical does not have the hazard characteristic, adverse effects mediated by an endocrine mode of action.
- Otherwise–
- confirmation that the chemical is not on the list of chemicals with high hazards for categorisation, based on its adverse effects mediated by an endocrine mode of action and
- confirmation that the chemical is not an ester or salt of the specified chemicals shown in the below table, which are on the list of chemicals with high hazards for categorisation, based on adverse effects mediated by an endocrine mode of action.
Adverse effects mediated by an endocrine mode of action - exception criteria
Check the following table - an ester or salt of the chemical has the adverse effects mediated by an endocrine mode of action hazard characteristic, unless one or more of the following exception criteria apply.
| CAS number | Chemical name | An ester or salt of the chemical has the adverse effects mediated by an endocrine mode of action hazard characteristic, unless one or more of the below exception criteria apply |
|---|---|---|
| 80-05-7 | Phenol, 4,4'-(1-methylethylidene)bis- (Bisphenol A) |
|
| 80-09-1 | Phenol, 4,4'-sulfonylbis- (Bisphenol S) |
|
| Various | Dodecylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
Note – Low levels of low molecular weight species (in relation to a polymer) means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol.
Genetic toxicity
Genetic toxicity means that any of the following apply to the industrial chemical:
- the chemical is known to induce or may induce mutations in the germ cells of humans, as described in chapter 3.5 of the GHS, with the chemical classified as germ cell mutagenicity (category 1 or 2), or
- the chemical is on the list of chemicals with high hazards for categorisation, based on its genetic toxicity, or
- the chemical is an ester or salt of specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation based on genetic toxicity unless an exception, as identified in the table below, is met for that chemical, or
- an in vitro study on the chemical:
- conducted following an acceptable test guideline for gene mutation or chromosomal abnormalities results in the prediction of mutagenic or genotoxic effects, as described in chapter 3.5 of the GHS, and
- the results of the study have not been negated by in vivo studies conducted on the chemical for gene mutation, chromosomal abnormalities or heritable germ cell mutagenicity, or
- an in vivo study on the chemical conducted following an acceptable test guideline for gene mutation, chromosomal abnormalities or heritable germ cell mutagenicity results in mutagenic or genotoxic effects, as described by chapter 3.5 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, genetic toxicity
The information required to demonstrate that a chemical does not have the hazard characteristic, genetic toxicity, is:
- if the human health exposure band for the introduction is 4 - at least one of the following:
- information to demonstrate that the chemical is included on the Select Committee on GRAS Substances (SCOGS) Database as a Type 1 conclusion, and that the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information to demonstrate that the chemical has been notified to the US FDA GRAS notification program and FDA had no questions about the notifier’s conclusion of GRAS status, and that the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- information to demonstrate that the chemical is a high molecular weight polymer, and if you are seeking to demonstrate that the introduction meets the criteria for very low risk and is not one of the 'special cases' mentioned in step 4.5 - test results from an in vitro study on the polymer or from suitable read across information conducted following an acceptable test guideline for gene mutation, which demonstrates the absence of mutagenic effects, or
- test results that demonstrate the absence of mutagenic or genotoxic effects from both:
- study on the chemical or from suitable read across information conducted following an acceptable test guideline for gene mutation, and
- study on the chemical or from suitable read across information conducted following an acceptable test guideline for chromosomal abnormalities.
- if the human health exposure band for the introduction is 3, and you are seeking to demonstrate that the introduction meets the criteria for very low risk and is not one of the 'special cases' mentioned in step 4.5 - at least one of the following:
- inclusion of the chemical in the Select Committee on GRAS Substances (SCOGS) Database as a Type 1 conclusion, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical has been notified to the US FDA GRAS notification program and FDA had no questions about the notifier’s conclusion of GRAS status, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- if the polymer is a high molecular weight polymer, test results from an in vitro study on the polymer or from suitable read across information conducted following an acceptable test guideline for gene mutations, which demonstrates the absence of mutagenic effects, or
- information that demonstrates the absence of mutagenic or genotoxic effects from both:
- information on the chemical or from suitable read across information that addresses gene mutations - this could be:
- a suitable in silico prediction, both with and without metabolic activation, or
- test results from a study conducted following an acceptable test guideline for gene mutations; and
- test results from a study on the chemical or from suitable read across information conducted following an acceptable test guideline for chromosomal abnormalities.
- information on the chemical or from suitable read across information that addresses gene mutations - this could be:
- Otherwise–
- confirmation that the chemical is not on the list of chemicals with high hazards for categorisation, based on its genetic toxicity and
- confirmation that the chemical is not an ester or salt of the specified chemicals shown in the below table, which are on the list of chemicals with high hazards for categorisation, based on genetic toxicity.
Note – Low levels of low molecular weight species (in relation to a polymer) means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol.
Genetic toxicity - exception criteria
Check the following table - an ester or salt of the chemical has the genetic toxicity hazard characteristic, unless one or more of the following exception criteria apply.
| CAS No | Chemical name | An ester or salt of the chemical has the genetic toxicity hazard characteristic, unless one or more of the below exception criteria apply |
|---|---|---|
| 90-43-7 | [1,1’-Biphenyl]-2-ol |
|
| 615-05-4 | 1,3-Benzenediamine, 4-methoxy- (diaminoanisole) |
|
| 123-30-8 | Phenol, 4-amino- |
|
| 95-55-6 | Phenol, 2-amino- |
|
- in addition, if the human health exposure band for the introduction is 4 and the chemical is a UV filter, information is required to justify why the chemical would not cause genetic toxicity mediated by UV light. This may include one or more of the following:
- the chemical has a molar extinction coefficient/absorption coefficient of less than 1,000 Lmol-1cm-1 at wavelengths between 290 and 700 nm (based on the results of a study following OECD test guideline 101), or
- results from in vitro phototoxicity studies, or
- results from in vitro or in vivo genetic toxicity studies where the methods have been modified to include photoactivation.
More information: Categorisation of UV filters
Examples - checking exception criteria for esters or salts
Example 1 – chemical is a salt of a specified chemical which is on the list of chemicals with high hazards for categorisation, based on carcinogenicity
Peter wants to introduce the chemical glycine, N,N-bis[2-[bis(carboxymethyl)amino]ethyl]-, sodium salt (1:?) (CAS: 7578-43-0), which is a salt of Glycine, N,N-bis(carboxymethyl)- (CAS: 139-13-9) that is in the table of specified chemicals for carcinogenicity. The proposed introduction has a molecular weight of 415.33 g/mol and therefore does not meet the exception criterion “the salt/ester is a high molecular weight polymer, with low levels of low molecular weight species” nor the exception criterion “the molecular weight of the salt/ester is greater than or equal to 1,000 g/mol”. Thus, Glycine, N,N-bis(2-(bis(carboxymethyl)amino)ethyl)-, sodium salt (CAS: 7578-43-0) is considered to have the human health hazard characteristic carcinogenicity.
Peter’s introduction's human health hazard band is C so he should move on to the next step – step 4.5 Work out your human health risk for categorisation.
Example 2 – chemical is an ester of a specified chemical which is on the list of chemicals with high hazards for categorisation, based on carcinogenicity and genetic toxicityRose wants to introduce an ester of [1,1’-Biphenyl]-2-ol, which has a molecular weight greater than or equal to 1,000 g/mol. The ester itself is not on the List, but her chemical is an ester of [1,1’-Biphenyl]-2-ol, which is in the tables of specified chemicals for carcinogenicity and genetic toxicity. So she needs to consider whether an exception applies to this chemical. If an exception does not apply, the ester would be considered to have the same high hazard characteristics as [1,1’-Biphenyl]-2-ol (carcinogenicity and genetic toxicity). But because Rose’s chemical has a molecular weight greater than or equal to1,000 g/mol, it meets the exception criteria.
Rose’s ester is not considered to have the human health hazard characteristics of carcinogenicity and genetic toxicity.
Rose continues through step 4.4 of the categorisation process and next needs to follow our guidance on checking human health hazard band B hazard characteristics.
Example 3 – polymer is an ester of a specified chemical which is on the list of chemicals with high hazards for categorisation, based on genetic toxicityPaul wants to introduce a branched polymer containing phenol, 2-amino- bound to side chains via ester linkages.
The polymer itself is not on the List, but it contains phenol, 2-amino-, which is in the table of specified chemicals for genetic toxicity. So he needs to consider whether an exception applies to this chemical. If an exception does not apply, the polymer would be considered to have the same high hazard characteristics as phenol, 2-amino- (genetic toxicity).The number average molecular weight (NAMW) of Paul’s polymer is 4,500 g/mol with 17% by mass having a molecular weight less than 1,000 g/mol and 8% by mass having a molecular weight less than 500 g/mol. The exception criteria for polymers states that there must be low levels of low molecular weight species which means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol. Therefore, the polymer meets the exception criteria and is not considered to have the genetic toxicity hazard characteristic.
Paul continues through step 4.4 of the categorisation process and next needs to follow our guidance on checking human health hazard band B hazard characteristics.
Human health hazard band B hazard characteristics
This page accompanies step 4.4 Work out your human health hazard characteristics.
Human health hazard characteristics are split into hazard bands. Hazard characteristics of most concern are in hazard band C, while those of lower concern are in hazard band A. You must always start at hazard band C. Step 4.4 tells you when you can stop working through your chemical's human health hazard characteristics and when you need to check each of them - ie C, B and A.
Hazard band B has 9 hazard characteristics you need to consider:
- High molecular weight polymer that is water absorbing
- Respiratory sensitisation
- Corrosive to the respiratory tract
- Specific target organ toxicity and a single exposure (significant toxicity)
- Skin corrosion
- Eye damage
- Skin sensitisation
- Acute toxicity (fatal or toxic)
- Specific target organ toxicity after repeated exposure
Instructions
You must always start at hazard band C.
You only need to work through the hazard characteristics on this page if your introduction is in:
- human health exposure band 3, and you are trying to get to an outcome of very low indicative human health risk or
- human health exposure band 4, and you are trying to get to an outcome of low or very low indicative human health risk.
Work your way through each hazard characteristic on this page. Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there - your introduction's human health hazard band is B. Move on to the next step - step 4.5 Work out your human health risk for categorisation.
If it does not meet the hazard characteristic definition, you’ll need to try and prove that your chemical does not have this hazard characteristic. The information that you need to prove this for each hazard characteristic is shown below. If you do not have this information, stop there - your introduction’s human health hazard band is B. Move onto the next step – step 4.5 Work out your human health risk for categorisation.
If you do have this information (so you can prove that the chemical does not have the hazard characteristic), move onto the next hazard characteristic on this page.
After you have considered all the hazard characteristics on this page and have proven that the chemical does not have any of them, decide whether you can stop there or continue to human health hazard band A This depends on the exposure band of your introduction.
If your introduction is in human health exposure band 3, continue to human health hazard band A.
If your introduction is in human health exposure band 4, you can choose to stop (and go to step 4.5 to work out your human health risk for categorisation), or continue to human health hazard band A.
High molecular weight polymer that is water absorbing
High molecular weight polymer that is water absorbing means that all of the following apply to the industrial chemical:
- it is a high molecular weight polymer, and
- it has a number average molecular weight that is greater than or equal to 10,000 g/mol, and
- it is capable of absorbing its own weight, or more, in water, and
- it contains particles with a particle size less than 10 micrometres (microns).
Information required to demonstrate the absence of the hazard characteristic, high molecular weight polymer that is water absorbing
If the chemical is a high molecular weight polymer, the information required to demonstrate that it does not have the hazard characteristic, high molecular weight polymer that is water absorbing, is at least one of:
- molecular weight information that demonstrates the number average molecular weight (NAMW) is less than 10,000 g/mol, or
- information that demonstrates that the polymer is not introduced in a particulate form, or
- particle size information that demonstrates that the particle size is greater than or equal to 10 micrometres (microns), or
- information that demonstrates that the polymer does not absorb its own weight or more in water, such as experiments that show that it does not form a gel in water, or that if it does, the gel dissolves upon addition of more water.
Respiratory sensitisation
Respiratory sensitisation means that any of the following apply to the industrial chemical:
- the chemical is known or presumed to produce hypersensitivity of the airways in humans, as described in chapter 3.4 of the GHS, with the chemical classified as respiratory sensitisation (category 1), or
- the chemical is named:
- on the EU SVHC Candidate list for authorisation, based on respiratory sensitising properties (https://echa.europa.eu/candidate-list-table), or
- in the Danish EPA (Q)SAR Database as a predicted respiratory sensitiser (http://qsar.food.dtu.dk/), or
- the chemical is an enzyme, or
- the chemical is a polymer that contains one or more free isocyanate groups, or
- an in vivo study on the chemical indicates hypersensitivity of the airways, as discussed in chapter 3.4 of the GHS, or
- an in vitro study on the chemical:
- indicates hypersensitivity of the airways, as discussed in chapter 3.4 of the GHS, and
- the result of the study has not been negated by in vivo studies conducted on the chemical for hypersensitivity of the airways.
Information required to demonstrate the absence of the hazard characteristic, respiratory sensitisation
There are no information requirements to demonstrate the absence of the hazard characteristic, respiratory sensitisation. If you do not have any of the information listed above that demonstrates that the chemical has this hazard characteristic then you can assume it does not for the purposes of categorisation.
Note: The information can include that the chemical is an enzyme or a polymer that contains one or more free isocyanate groups.
Corrosive to the respiratory tract
Corrosive to the respiratory tract means that any of the following apply to the industrial chemical:
- the chemical is known to cause destruction of the respiratory tract tissue, as described in chapter 3.1 of the GHS, with the chemical classified as corrosive to the respiratory tract (AUH071 - non-GHS hazard statement), or
- an in vivo study on the chemical conducted following an acceptable test guideline for acute inhalation toxicity, subacute inhalation toxicity or subchronic inhalation toxicity results in destruction of the respiratory tract, as described in chapter 3.1 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, corrosive to the respiratory tract
There are no information requirements to demonstrate the absence of the hazard characteristic, corrosive to the respiratory tract. If you do not have any of the information listed above that demonstrates that the chemical has this hazard characteristic, then you can assume it does not for the purposes of categorisation.
Specific target organ toxicity after a single exposure (significant toxicity)
Specific target organ toxicity after a single exposure (significant toxicity) means that any of the following apply to the industrial chemical:
- the chemical is known or presumed to produce significant toxicity in humans, as described in chapter 3.8 of the GHS, with the chemical classified as specific target organ toxicity – single exposure (category 1), or
- an in vivo study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS, at less than or equal to 300 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS, at less than or equal to 1,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS:
- gases - less than or equal to 2,500 ppmV/4h, or
- vapours - less than or equal to mg/L/4h, or
- for dusts/mists/fumes - less than or equal to 1 mg/L/4h.
Information required to demonstrate the absence of the hazard characteristic, specific target organ toxicity after a single exposure (significant toxicity)
There are no information requirements to demonstrate the absence of the hazard characteristic, specific target organ toxicity after a single exposure (significant toxicity). If you do not have any of the information listed above that demonstrates that the chemical has this hazard characteristic, then you can assume it does not for the purposes of categorisation.
Skin corrosion
Skin corrosion means that any of the following apply to the industrial chemical:
- the chemical is known to produce irreversible damage to the skin, as described in chapter 3.2 of the GHS, with the chemical classified as skin corrosion (category 1), or
- an in vitro study on the chemical conducted following an acceptable test guideline for skin corrosion results in the prediction of skin corrosion effects, or
- an in vivo study on the chemical conducted following an acceptable test guideline for skin irritation results in destruction of skin tissue, as described for skin corrosion in chapter 3.2 of the GHS, or
- the chemical is a pyrophoric liquid or a pyrophoric solid, as described in chapters 2.9 and 2.10 of the GHS respectively.
Information required to demonstrate the absence of the hazard characteristic, skin corrosion
The information required to demonstrate that a chemical does not have the hazard characteristic, skin corrosion, is at least one of the following:
- if the human health exposure band for the introduction is 3 or 4
- information that demonstrates that the chemical is a high molecular weight polymer that does not contain any of the following reactive functional groups:
- anhydride, or
- epoxide, or
- sulfonic acid, or
- amine, or
- information that demonstrates that the chemical is a high molecular weight polymer that contains any of the following reactive functional groups and the polymer has a combined functional group equivalent weight of greater than or equal to 1,000 g/mol:
- anhydride, or
- epoxide, or
- sulfonic acid, or
- amine, or
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin corrosion, with a non-corrosive prediction, or
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin irritation, with a non-irritant prediction, or
- test results from an in vivo study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin irritation, which does not result in destruction of skin tissue, as described for skin corrosion (category 1) in chapter 3.2 of the GHS.
- information that demonstrates that the chemical is a high molecular weight polymer that does not contain any of the following reactive functional groups:
- if the human health exposure band for the introduction is 3 – a suitable in silico prediction indicating that the chemical is not irritating to skin or has no alerting groups for skin irritation.
Eye damage
Eye damage means that any of the following apply to the industrial chemical:
- the chemical is known to produce serious eye damage, as described in chapter 3.3 of the GHS, with the chemical classified as eye damage (category 1), or
- an in vitro study on the chemical conducted following an acceptable test guideline for eye damage results in the prediction of serious eye damage effects, or
- an in vivo study on the chemical conducted following an acceptable test guideline for eye irritation results in effects on the eye, as described for serious eye damage in chapter 3.3 of the GHS, or
- the chemical is a pyrophoric liquid or a pyrophoric solid, as described in chapters 2.9 and 2.10 of the GHS, respectively.
Information required to demonstrate the absence of the hazard characteristic, eye damage
The information required to demonstrate that a chemical does not have the hazard characteristic, eye damage, is at least one of the following:
- if the human health exposure band for the introduction is 3 or 4
- information that demonstrates that the chemical is a high molecular weight polymer that does not contain any of the following reactive functional groups:
- anhydride, or
- epoxide, or
- sulfonic acid, or
- amine, or
- information that demonstrates that the chemical is a high molecular weight polymer that contains any of the following reactive functional groups with a combined functional group equivalent weight of greater than or equal to 1,000 g/mol:
- anhydride, or
- epoxide, or
- sulfonic acid, or
- amine, or
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for eye damage, which predicts the chemical would not induce serious eye damage
- test results from an in vivo study on the chemical or from suitable read across information, conducted following an acceptable test guideline for eye irritation, which does not result in effects on the eye, as described for eye damage in chapter 3.3 of the GHS.
- information that demonstrates that the chemical is a high molecular weight polymer that does not contain any of the following reactive functional groups:
- if the human health exposure band for the introduction is 3 - a suitable in silico prediction indicating that the chemical is not irritating to the eye.
Skin sensitisation
Skin sensitisation means that any of the following apply to the industrial chemical:
- the chemical is known to cause an allergic response following skin contact, as described in chapter 3.4 of the GHS, with the chemical classified as skin sensitisation (category 1), or
- human testing or epidemiological studies on the chemical result in evidence of an allergic response, as described in chapter 3.4 of the GHS, or
- an in vitro study on the chemical:
- conducted following an acceptable test guideline for skin sensitisation, results in the prediction of skin sensitisation effects, and
- the results of the study have not been negated by in vivo studies conducted on the chemical for skin sensitisation, or
- an in chemico study on the chemical:
- conducted following an acceptable test guideline for skin sensitisation, results in the prediction of skin sensitisation effects, and
- the results of the study have not been negated by in vivo studies conducted on the chemical for skin sensitisation, or
- an in vivo study on the chemical conducted following an acceptable test guideline for skin sensitisation, results in the induction of an allergic response, as described in chapter 3.4 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, skin sensitisation
- There are no information requirements to demonstrate the absence of the hazard characteristic, skin sensitisation, if the chemical is corrosive to the skin (GHS category 1) as the in vivo tests cannot be conducted
- otherwise, if the human health exposure band is 3 or 4, the information required to demonstrate that a chemical does not have the hazard characteristic, skin sensitisation, is at least one of the following:
- information that demonstrates that the chemical is a high molecular weight polymer for which at least one of the following applies:
- it contains only low concern reactive functional groups, or
- it contains only low concern reactive functional groups and unsubstituted positions ortho and para to phenolic hydroxyl groups, or
- the only reactive functional groups it contains are unsubstituted positions ortho and para to phenolic hydroxyl groups, or
- it contains only low and moderate concern reactive functional groups, with a combined functional group equivalent weight for the moderate-concern functional groups of greater than or equal to 1000 g/mol, or
- it contains only moderate concern reactive functional groups, with a combined functional group equivalent weight of greater than or equal to 1000 g/mol, or
- it has a number average molecular weight that is greater than or equal to 10,000 g/mol and both:
- less than 2% by mass of molecules with molecular weight that is less than 500 g/mol, and
- less than 5% by mass of molecules with molecular weight that is less than 1,000 g/mol, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V, of the REACH Regulation, or
- results from a defined approach (combination of tests) described in OECD TG 497, with a non-sensitising prediction*, or
- all of the following:
- test results from an in chemico test on the chemical or from suitable read-across information, conducted following an acceptable test guideline for the 1st key event in the adverse outcome pathway for skin sensitisation, with a non-sensitising prediction, and
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for the 2nd key event in the adverse outcome pathway for skin sensitisation, with a non-sensitising prediction, and
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for the 3rd key event in the adverse outcome pathway for skin sensitisation, with a non-sensitising prediction
- test results from an in vivo study on the chemical or from suitable read-across information, conducted following an acceptable test guideline for skin sensitisation, which does not result in induction of an allergic response, as described in chapter 3.4 of the GHS
- information that demonstrates that the chemical is a high molecular weight polymer for which at least one of the following applies:
- in addition, if the human health exposure band for the introduction is 4 and the chemical is a UV filter or is introduced for an end use in a tattoo ink, information is required to justify why the chemical would not cause skin sensitisation mediated by UV light. This may include one or more of the following :
- the chemical has a molar extinction coefficient/absorption coefficient of less than 1,000Lmol-1cm-1 at wavelengths between 290 and 700nm (based on the results of a study following OECD test guideline 101), or
- results from in vitro phototoxicity studies, or
- results from in vitro or in vivo skin sensitisation studies where the methods have been modified to include photoactivation.
Low concern reactive functional groups – see our polymer of low concern criteria page.
Moderate concern reactive functional groups – see our polymer of low concern criteria page.
*Note that negative results only with key event 1 and key event 2 adverse outcome pathway (AOP) assays will not rule out skin sensitisation potential for some weak skin sensitisers and pre-/pro-hapten fragrance chemicals when using the "2 out of 3" (2o3) defined approach and hence this should be considered when determining an appropriate testing methodology. For further information see: ENV/CBC/MONO(2021)11. Supporting Document to the OECD Guideline 497 on Defined Approaches for Skin Sensitisation
Acute toxicity (fatal or toxic)
Acute toxicity (fatal or toxic) means that any of the following apply to the industrial chemical:
- the chemical is known to exhibit acute toxicity effects, as described in chapter 3.1 of the GHS, with the chemical classified as acute toxicity (category 1 or 2 or 3), or
- an in vitro study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity, results in a predicted acute oral toxicity LD50 value of less than or equal to 300 mg/kg bw, and
- the results of the study have not been negated by in vivo studies conducted on the chemical for acute toxicity, or
- an in vivo study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity results in an acute oral LD50 value of less than or equal to 300 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity results in an acute dermal LD50 value of less than or equal to 1,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity results in an acute inhalation LC50 value of:
- for gases - less than or equal to 2,500 ppmV/4h, or
- for vapours - less than or equal to 10 mg/L/4h, or
- for dusts/mists/fumes - less than equal to 1 mg/L/4h , or
- evidence in humans of systemic toxicity after eye contact, with the chemical classified with the non-GHS hazard statement – AUH070, or
- an in vivo study, conducted following an acceptable test guideline for eye irritation, results in overt signs of systemic toxicity or mortality, which is likely to be attributed to absorption of the chemical through the mucous membranes of the eye, with the chemical classified with the non-GHS hazard statement – AUH070.
Information required to demonstrate the absence of the hazard characteristic, acute toxicity (fatal or toxic)
- there are no information requirements to demonstrate the absence of the hazard characteristic, acute toxicity (fatal or toxic), if the chemical is corrosive to the skin (GHS category 1), or likely to be corrosive to the skin (that is, the chemical is a strong acid (pH less than or equal to 2.0) or base (pH greater than or equal to 11.5), and has high buffering capacity (if relevant)), as the in vivo tests cannot be conducted
- otherwise, the information required to demonstrate that a chemical does not have the hazard characteristic, acute toxicity (fatal or toxic), is at least one of the following:
- if the human health exposure band for the introduction is 3 – both of the following:
- a suitable in silico prediction for acute toxicity (LD50) of the chemical of greater than 2,000 mg/kg bw/day, and
- test results from an in vitro study on the chemical or from suitable read across information for acute toxicity (LD50), conducted following an acceptable test guideline for acute oral toxicity, of greater than 300 mg/kg bw, or
- if the human health exposure band for the introduction is 3 or 4
- information that demonstrates that the chemical is a high molecular weight polymer that has:
- less than 5% by mass of molecules with molecular weight less than 1,000 g/mol, and
- less than 2% by mass of molecules with molecular weight less than 500 g/mol, or
- information that demonstrates that the chemical is a high molecular weight polymer that has:
- inclusion of the chemical in the Select Committee on GRAS Substances (SCOGS) Database as a Type 1 conclusion, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical has been notified to the US FDA GRAS notification program and FDA had no questions about the notifier’s conclusion of GRAS status, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical is permitted to be used as a food additive according to Schedule 15 of the Australia New Zealand Food Standards Code - Standard 1.3.1 - Food Additives, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- a test result from at least one in vivo study on the chemical or from suitable read across information, as detailed below, with the administration route dependent on the most relevant route of exposure (or the oral route if information on the most relevant route is not available):
- conducted following an acceptable test guideline for acute oral toxicity with an LD50 greater than 300 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity with an LD50 greater than 1,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity, with an LC50:
- for gases - greater than 2,500 ppmV/4h, or
- for vapours - greater than 10 mg/L/4h, or
- for dusts/mists/fumes - greater than 1 mg/L/4h, or
- test results from an in vivo study via the oral route on the chemical or from suitable read across information, conducted following an acceptable test guideline for subacute oral toxicity, with a NOAEL greater than or equal to 1,000 mg/kg bw/day.
- if the human health exposure band for the introduction is 3 – both of the following:
Specific target organ toxicity after repeated exposure
Specific target organ toxicity after repeated exposure means that any of the following apply to the industrial chemical:
- the chemical is known to exhibit significant toxicity or be potentially harmful to human health following repeated exposure, as described in chapter 3.9 of the GHS, with the chemical classified as specific target organ toxicity – repeated exposure (category 1 or 2), or
- an in vivo study on the chemical:
- conducted following an acceptable test guideline for subacute oral toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEL (oral) value of less than 300 mg/kg bw/day, or
- conducted following an acceptable test guideline for subchronic oral toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEL (oral) of less than 100 mg/kg bw/day, or
- conducted following an acceptable test guideline for subacute dermal toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEL (dermal) value of less than 600 mg/kg bw/day, or
- conducted following an acceptable test guideline for subchronic dermal toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEL (dermal) of less than 200 mg/kg bw/day, or
- conducted following an acceptable test guideline for subacute inhalation toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEC (inhalation) of:
- for gases - less than 750 ppmV/6 h/day, or
- for vapours - less than 3 mg/L/6 h/day, or
- for dusts/mists/fumes less than 0.6 mg/L/6 h/day, or
- conducted following an acceptable test guideline for subchronic inhalation toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEC (inhalation) of:
- for gases - less than 250 ppmV/6 h/day, or
- for vapours - less than 1 mg/L/6 h/day, or
- for dusts/mists/fumes less than 0.2 mg/L/6 h/day.
Information required to demonstrate the absence of the hazard characteristic, specific target organ toxicity after repeated exposure
Information is required to demonstrate the absence of the hazard characteristic, specific target organ toxicity after repeated exposure, if:
- the human health exposure band for the introduction is 4, and
- you are seeking to demonstrate that the introduction meets the criteria for low risk or very low risk
and any of the following apply:
- the human health categorisation volume for the introduction is greater than 100 kg and the chemical is introduced for end use in any of the following types of articles:
- food contact, or
- children’s toys that can be placed in the mouth, or
- children’s care products that can be placed in the mouth, or
- the human health categorisation volume for the introduction is greater than 1000 kg and the chemical is not introduced for end use in any of the following types of articles:
- food contact, or
- children’s toys that can be placed in the mouth, or
- children’s care products that can be placed in the mouth.
See extra guidance on categorisation of chemicals:
If 1 or 2 apply, the information required to demonstrate that a chemical does not have the hazard characteristic, specific target organ toxicity after repeated exposure, is at least one of the following:
- inclusion of the chemical in the Select Committee on GRAS Substances (SCOGS) Database as a Type 1 conclusion, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical has been notified to the US FDA GRAS notification program and FDA had no questions about the notifier’s conclusion of GRAS status, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical is permitted to be used as a food additive according to Schedule 15 of the Australia New Zealand Food Standards Code - Standard 1.3.1 - Food Additives, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- information that demonstrates that the chemical is a high molecular weight polymer that has
- < 5% by mass of molecules with molecular weight < 1000 g/mol, and
- < 2% by mass of molecules with molecular weight < 500 g/mol, or
- a test result from at least one in vivo study on the chemical or from suitable read across information, as detailed below, with the administration route dependent on the most relevant route of exposure (or the oral route if information on the most relevant route is not available):
- conducted following an acceptable test guideline for subacute oral toxicity, in which the NOAEL (oral) is greater than or equal to 300 mg/kg bw/day or there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or
- conducted following an acceptable test guideline for subchronic oral toxicity, in which the NOAEL (oral) is greater than or equal to 100 mg/kg bw/day or there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or
- conducted following an acceptable test guideline for subacute dermal toxicity, in which the NOAEL (dermal) is greater than or equal to 600 mg/kg bw/day or there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or
- conducted following an acceptable test guideline for subchronic dermal toxicity, in which the NOAEL (dermal) is greater than or equal to 200 mg/kg bw/day or there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or
- conducted following an acceptable test guideline for subacute inhalation toxicity, in which there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or the NOAEC (inhalation) is:
- for gases - greater than or equal to 750 ppmV/6 h/day, or
- for vapours - greater than or equal to 3 mg/L/6 h/day, or
- for dusts/mists/fumes - greater than or equal to 0.6 mg/L/6 h/day , or
- conducted following an acceptable test guideline for subchronic inhalation toxicity, in which there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or the NOAEC (inhalation) is:
- for gases - greater than or equal to 250 ppmV/6 h/day, or
- for vapours - greater than or equal to 1 mg/L/6 h/day, or
- for dusts/mists/fumes - greater than or equal to 0.2 mg/L/6 h/day.
There are no information requirements to demonstrate the absence of the hazard characteristic, specific target organ toxicity after repeated exposure, if:
- the human health exposure band for the introduction is 4, and
- you are seeking to demonstrate that the introduction meets the criteria for low risk or very low risk,
and any of the following apply:
- the human health categorisation volume for the introduction is less than or equal to 100 kg and the chemical is introduced for end use in any of the following types of articles:
- food contact, or
- children’s toys that can be placed in the mouth, or
- children’s care products that can be placed in the mouth, or
- the human health categorisation volume for the introduction is less than or equal to 1,000 kg and the chemical is not introduced for end use in any of the following types of articles:
- food contact, or
- children’s toys that can be placed in the mouth, or
- children’s care products that can be placed in the mouth
In these circumstances, if you do not have any information that demonstrates that the chemical has this hazard characteristic then you can assume it does not for the purposes of categorisation.
Human health hazard band A hazard characteristics
Human health hazard characteristics are split into hazard bands. Hazard characteristics of most concern are in hazard band C, while those of lower concern are in hazard band A.
Hazard band A has 6 hazard characteristics you need to consider:
- High molecular weight polymer that has lung overloading potential
- Aspiration hazard
- Specific target organ toxicity after a single exposure (harmful or transient effects)
- Skin irritation
- Eye irritation
- Acute toxicity (harmful)
Instructions
You must always start at hazard band C. You only need to work through the hazard characteristics on this page if your introduction is in:
- human health exposure band 3, and you are trying to get to an outcome of very low indicative human health risk or
- human health exposure band 4, and you are trying to get to an outcome of very low indicative human health risk.
Work your way through each hazard characteristic on this page. Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there - your introduction's human health hazard band is A.
Move on to the next step - step 4.5 Work out your human health risk for categorisation.
If it does not meet the hazard characteristic definition, you’ll need to try and prove that your chemical does not have this hazard characteristic. The information that you need to prove this for each hazard characteristic is shown below. If you do not have this information, stop there - your introduction’s human health hazard band is A.
Move onto the next step – step 4.5 Work out your human health risk for categorisation.
If you do have this information (so you can prove that the chemical does not have the hazard characteristic), move onto the next hazard characteristic on this page.
After you have considered all the hazard characteristics on this page and have proven that the chemical does not have any of them, go to step 4.5 to work out your human health risk for categorisation).
High molecular weight polymer that has lung overloading potential
This page accompanies step 4.4 Work out your human health hazard characteristics.
High molecular weight polymer that has lung overloading potential means that all of the following apply to the industrial chemical:
- it is a polymer, and
- it has a number average molecular weight that is greater than 70,000 g/mol, and
- it has a solubility in water of less than 0.1 mg/L, and
- it becomes aerosolised during end use.
Information required to demonstrate the absence of the hazard characteristic, high molecular weight polymer that has lung overloading potential
If the chemical is a polymer, the information required to demonstrate that a chemical does not have the hazard characteristic, high molecular weight polymer that has lung overloading potential, is at least one of the following:
- molecular weight information that demonstrates that the number average molecular weight is less than or equal to 70,000 g/mol, or
- information that demonstrates that the polymer has a solubility in water that is greater than or equal to 0.1 mg/L measured following an acceptable test guideline for water solubility, or
- information that demonstrates that the polymer does not become aerosolised during end use
Aspiration hazard
Aspiration hazard means that any of the following apply to the industrial chemical:
- the chemical is known or presumed to cause aspiration toxicity, as described in chapter 3.10 of the GHS, with the chemical classified as may be fatal if swallowed and enters airways (category 1), or
- the chemical is a hydrocarbon that has a kinematic viscosity less than or equal to 20.5 mm2/s, measured at 40°C.
Information required to demonstrate the absence of the hazard characteristic, aspiration hazard
- If the chemical is a hydrocarbon, the information required to demonstrate the absence of the hazard characteristic, aspiration hazard, is a measured kinematic viscosity greater than a 20.5mm2/s, at 40 °C
- Otherwise, there are no information requirements to demonstrate the absence of the hazard characteristic, aspiration hazard. If you do not have any information that demonstrates that the chemical has this hazard characteristic, then you can assume it does not for the purposes of categorisation.
Specific target organ toxicity after a single exposure (harmful or transient effects)
Specific target organ toxicity after a single exposure (harmful or transient effects) means that any of the following apply to the industrial chemical:
- the chemical is known or presumed to be harmful to humans or to cause transient target organ effects, as described in chapter 3.8 of the GHS, with the chemical classified as specific target organ toxicity-single exposure (category 2 or 3), or
- an in vivo study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS, at greater than 300 but less than or equal to 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS, at greater than 1,000 but less than or equal to 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS, at:
- for gases - greater than 2,500 but less than or equal to 20,000 ppmV/4h, or
- for vapours - greater than 10 but less than or equal to 20 mg/L/4h, or
- for dusts/mists/fumes - greater than 1 but less than or equal to 5 mg/L/4h.
Information required to demonstrate the absence of the hazard characteristic, specific target organ toxicity after a single exposure (harmful or transient effects)
There are no information requirements to demonstrate the absence of the hazard characteristic, specific target organ toxicity after a single exposure (harmful or transient effects). If you do not have any information that demonstrates that the chemical has this hazard characteristic, then you can assume it does not for the purposes of categorisation.
Skin irritation
Skin irritation means that any of the following apply to the industrial chemical:
- the chemical is known to produce reversible damage to the skin, as described in chapter 3.2 of the GHS, with the chemical classified as skin irritation (category 2), or
- an in vitro study on the chemical conducted following an acceptable test guideline for skin irritation results in the prediction of skin irritation effects, or
- an in vivo study on the chemical conducted following an acceptable test guideline for skin irritation results in skin reactions, as described for skin irritation (category 2) in chapter 3.2 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, skin irritation
The information required to demonstrate that a chemical does not have the hazard characteristic, skin irritation, is at least one of the following:
- if the human health exposure band for the introduction is 3 - a suitable in silico prediction indicating that the chemical is not irritating to the skin, or
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin irritation, with a non-irritant prediction, or
- if the human health exposure band for the introduction is 3 or 4
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin irritation, with a non-irritant prediction, or
- test results from an in vivo study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin irritation, which does not result in skin reactions, as described for skin irritation (category 2) in chapter 3.2 of the GHS, or
- test results from an in vivo study on the chemical or from suitable read across information conducted following an acceptable test guideline for acute dermal toxicity, that when tested at 2,000 mg/kg bw/day is not irritating to the skin.
In addition, if the chemical is introduced for an end use in a tattoo ink, information is required to justify why the chemical would not cause skin irritation mediated by UV light. This may include one or more of the following:
- the chemical has a molar extinction coefficient/absorption coefficient of less than 1,000Lmol-1cm-1 at wavelengths between 290 and 700 nm (based on the results of a study following OECD test guideline 101), or
- results from in vitro phototoxicity studies, or
- results from in vitro or in vivo irritation studies where the methods have been modified to include photoactivation.
Eye irritation
Eye irritation means that any of the following apply to the industrial chemical:
- the chemical is known to produce changes in the eye, as described in chapter 3.3 of the GHS, with the chemical classified as eye irritation (category 2), or
- an in vitro study on the chemical conducted following an acceptable test guideline for eye irritation results in the prediction of eye irritation effects, or
- an in vivo study on the chemical conducted following an acceptable test guideline for eye irritation results in changes in the eye, as described for eye irritation in chapter 3.3 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, eye irritation
The information required to demonstrate that a chemical does not have the hazard characteristic, eye irritation, is at least one of the following:
- if the human health exposure band for the introduction is 3 - a suitable in silico prediction indicating that the chemical is not irritating to the eyes, or
- if the human health exposure band for the introduction is 3 or 4
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for eye damage or eye irritation with a non-irritant prediction, or
- test results from an in vivo study on the chemical or from suitable read across information, conducted following an acceptable test guideline for eye irritation, which does not result in changes in the eyes, as described for eye irritation (category 2) in chapter 3.3 of the GHS.
Acute toxicity (harmful)
Acute toxicity (harmful) means that any of the following apply to the industrial chemical:
- the chemical is known to exhibit acute toxicity effects, as described in chapter 3.1 of the GHS, with the chemical classified as acute toxicity (category 4), or
- an in vitro study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity, results in a predicted acute toxicity LD50 value of greater than 300 but less than or equal to 2,000 mg/kg bw, and
- the results of the study have not been negated by in vivo studies conducted on the chemical for acute toxicity, or
- an in vivo study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity results in an acute oral LD50 value of greater than 300 but less than or equal to 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity results in an acute dermal LD50 value of greater than 1,000 but less than or equal to 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity results in an acute inhalation LC50 value of:
- for gases - greater than 2500 but less than or equal to 20,000 ppmV/4h, or
- for vapours - greater than 10 but less than or equal to 20 mg/L/4h, or
- for dusts/mists/fumes - greater than 1 but less than or equal to 5 mg/L/4h.
Information required to demonstrate the absence of the hazard characteristic, acute toxicity (harmful)
- the information required to demonstrate that a chemical does not have the hazard characteristic, acute toxicity (harmful), is at least one of the following:
- if the human health exposure band for the introduction is 3 - both of the following:
- a suitable in silico prediction for acute toxicity (LD50) of the chemical of greater than 2,000 mg/kg bw/day, and
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for acute oral toxicity, with a predicted LD50 of greater than 2,000 mg/kg bw, or
- if the human health exposure band for the introduction is 3 or 4
- information that demonstrates that the chemical is a high molecular weight polymer that has:
- less than 5% by mass of molecules with molecular weight less than 1,000 g/mol, and
- less than 2% by mass of molecules with molecular weight less than 500 g/mol
- inclusion of the chemical in the Select Committee on GRAS Substances (SCOGS) Database as a Type 1 conclusion, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical has been notified to the US FDA GRAS notification program and FDA had no questions about the notifier’s conclusion of GRAS status, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical is permitted to be used as a food additive according to Schedule 15 of the Australia New Zealand Food Standards Code - Standard 1.3.1 - Food Additives, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- a test result from at least one in vivo study on the chemical or from suitable read across information, as detailed below, with the administration route dependent on the most relevant route of exposure (or the oral route if information on the most relevant route is not available):
- conducted following an acceptable test guideline for acute oral toxicity with an LD50 greater than 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity with an LD50 greater than 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity with an LC50:
- for gases - greater than 20,000 ppmV/4h, or
- for vapours - greater than 20 mg/L/4h, or
- for dusts/mists/fumes - greater than 5 mg/L/4h, or
- test results from an in vivo study on the chemical or from suitable read across information, conducted following an acceptable test guideline for subacute oral toxicity, with a NOAEL greater than or equal to 1,000 mg/kg bw/day.
- information that demonstrates that the chemical is a high molecular weight polymer that has:
- if the human health exposure band for the introduction is 3 - both of the following:
Step 4.5 Outcome - your human health risk for categorisation
Get help with this step — explore our categorisation decision tools
We explain the table in detail for each human health exposure band that your introduction could be in. This includes what your indicative human health risk outcome will be, depending on which hazard characteristics your chemical does or does not have. Your outcome will be that your introduction has an indicative human health risk of:
- medium to high
- low OR
- very low
Refer back to step 4.4 for information about how to consider the hazard characteristics and where to start and stop when considering hazard characteristics.
Human health risk table
| Work out your indicative human health risk | Human health exposure band | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Human health hazard band | C | Low risk | Medium to high risk | Medium to high risk | Medium to high risk |
| B | Very low risk | Very low risk | Low risk | Medium to high risk | |
| A | Very low risk | Very low risk | Low risk | Low risk | |
| Not C, B, A | Very low risk | Very low risk | Very low risk | Very low risk | |
If your introduction is in human health exposure band 1
If your introduction is in human health exposure band 1, you will need to consider if your chemical has any of the hazard characteristics in human health hazard band C. It’s not necessary to consider the hazard characteristics in band B or A.
The indicative human health risk of your introduction will be:
- low if your chemical has 1 or more of the hazard characteristics in human health hazard band C or
- very low if your chemical does not have any of the hazard characteristics in human health hazard band C
The table on this page shows how you can work out your indicative human health risk by using your human health exposure band and the human health hazard characteristics that your chemical does or does not have.
If your introduction is in human health exposure band 2
If your introduction is in human health exposure band 2, you will need to consider if your chemical has any of the hazard characteristics in human health hazard band C. It’s not necessary to consider the hazard characteristics in band B or A.
The indicative human health risk of your introduction will be:
- medium to high if your chemical has 1 or more of the hazard characteristics in human health hazard band C OR
- very low if your chemical does not have any of the hazard characteristics in human health hazard band C
If your introduction is in human health exposure band 3
If your introduction is in human health exposure band 3, at a minimum, you will need to consider if your chemical has any of the hazard characteristics in human health hazard band C.
The indicative human health risk of your introduction will be:
- medium to high if your chemical has 1 or more of the hazard characteristics in human health hazard band C OR
- low if your chemical does not have any of the hazard characteristics in human health hazard band C
You can choose to stop if you get to low indicative human health risk.
If you want to see if your introduction could have a very low indicative human health risk, you will also need to consider if it has any of the hazard characteristics in human health hazard bands B and A.
The indicative human health risk of your introduction will be:
- low if your chemical has 1 or more of the hazard characteristics in human health hazard band B or A or
- very low if your chemical does not have any of the hazard characteristics in human health hazard band B or A
If your introduction is in human health exposure band 4
If your introduction is in human health exposure band 4, you will first need to consider if your chemical has any of the hazard characteristics in human health hazard band C. If it does not, then continue on to consider the hazard characteristics in human health hazard band B.
The indicative human health risk of your introduction will be:
- medium to high if your chemical has 1 or more of the hazard characteristics in human health hazard band C or B or
- low if your chemical does not have any of the hazard characteristics in human health hazard band C or B
You can choose to stop if you get to low indicative human health risk.
If you want to see if your introduction could have a very low indicative human health risk, you will also need to consider if it has any of the hazard characteristics in human health hazard band A.
The indicative human health risk of your introduction will be:
- low if your chemical has 1 or more of the hazard characteristics in human health hazard band A or
- very low if your chemical does not have any of the hazard characteristics in human health hazard band A
'Special cases' — introductions that cannot have a very low indicative human health risk
Your introduction cannot have a very low indicative human health risk if it is a:
- UV filter or
- chemical that is introduced as a solid or a dispersion that is not soluble, that meets the nanoscale particle size criteria, and the introduction of the nanoscale portion of the chemical (the part that has a particle size range of 1 nm to 100 nm) is incidental to the introduction of the non-nanoscale portion or
- chemical that is introduced as a solid or a dispersion where there is no information available on its water solubility or its particle size, and the introduction of any nanoscale portion of the chemical (the part that has a particle size range of 1 nm to 100 nm) is incidental to the introduction of the non-nanoscale portion.
If your introduction is 1 of these, and you got a very low risk outcome in this step, you need to change that outcome to low risk.
This means if your consideration of step 4.5 got you to an outcome of very low risk, your final outcome needs to be changed to low risk.
Definitions of these 'special cases'
A UV filter is a chemical that is intended to protect the skin against ultraviolet radiation in the range of 290 nm to 400 nm by absorption, reflection, or scattering of ultraviolet radiation.
Nanoscale particle size criteria means that the chemical consists of solid particles in an unbound state or as an aggregate or agglomerate. At least 50% (by number size distribution) of the particles must have at least 1 external dimension in the particle size range of 1nm to 100nm (i.e. the nanoscale).
Not soluble means the solubility of the chemical in water is less than 33.3 g/L measured following an acceptable test guideline for water solubility; or the dissolution rate of the chemical is not more than 70%.
Next
Go to step 5 to work out the risk to the environment of your introduction